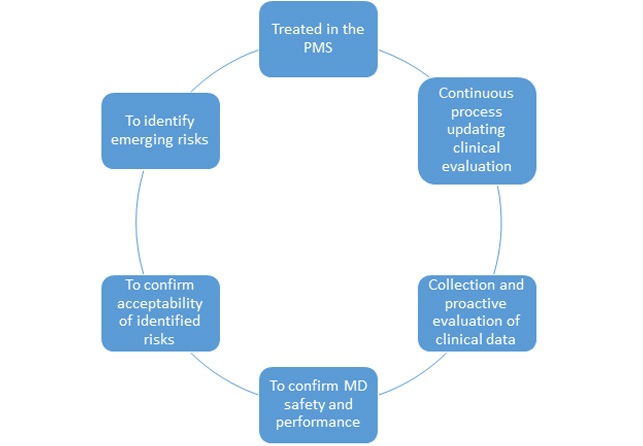
PMCF – Post Market Clinical Follow up
Along with establishing the PMCF as a requirement, Regulation (EU) 2017/745 establishes that this should be carried out according to a document method established in a plan.
Having their own group of experts, Di Renzo Regulatory Affairs provides the following services:
- definition and drafting of the PMCF plan
- drafting of the PMCF report
- performance of PMCF activities such as: search and analysis of clinical literature, search on registers and databases, drafting of users’ questionnaires
- support in the management of PMCF studies.
Foto di mohamed_hassan da Pixabay






