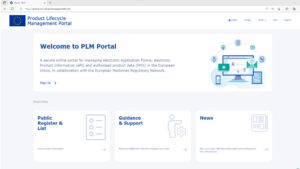
EU RA procedures go digital: the new application form format (eAF) and the new product data format (PMS) – update July 2023
The European Medicine Agency (EMA) is working on the digitalisation of regulatory processes for medicines with the aim of facilitating...